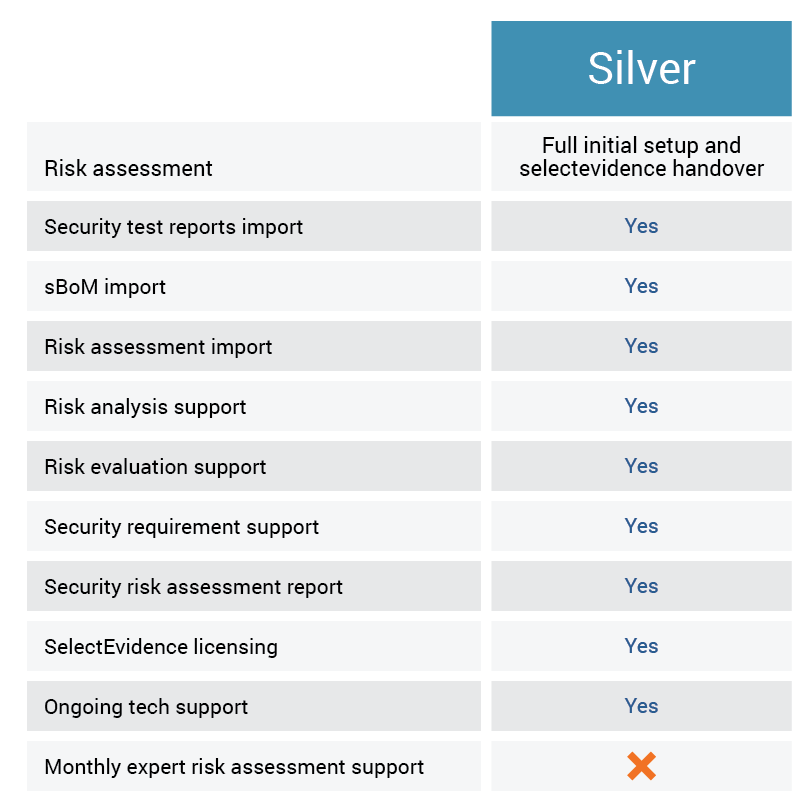
Selectevidence supports manufacturers in designing verifying and certifying medical devices to meet fda cybersecurity recommendations best practices for connected medical devices and industry security standards.
Medical device cybersecurity risk mitigation conference.
Healthcare and manufacturing are coming together to solve these challenges and this conference.
Medical devices and associated systems are especially vulnerable.
Why is the medical device cybersecurity risk mitigation conference important to medical device security and manufacturing teams.
Bob kolasky director national risk management center department of homeland security.
Cybersecurity collaboration between mdms hdos to ensure patient safety module 3.
Recent attacks on healthcare and multiple new vulnerabilities discovered highlight the need to make meaningful improvements.
Ensuring compliance with regulatory requirements and industry standards to mitigate cyber risks and continuously improve medical device security in addition to collaborating and sharing responsibility between health delivery organizations and manufacturers.
Security in the healthcare industry is in a higher risk state than almost any other industry.
This year s medical device cybersecurity risk mitigation conference will virtually connect security leaders to share best practices in the following modules.
Medical device security will not be solved individually.
Nova leah specialize in expert cybersecurity risk management software solutions for the medical device industry.
Healthcare is an ecosystem that medical devices play a role in.
Medical device manufacturers mdms and health care delivery organizations hdos should take steps to ensure appropriate safeguards are in place.
Managing cybersecurity risk through various assessments robust management systems.
4th annual medical device cybersecurity risk mitigation conference july 23 24 2019 arlington va.
9 30 initiatives to further medical device cybersecurity mitre is developing a rubric for the common vulnerability scoring system cvss to help medical device manufacturers mdms and healthcare delivery organizations hdos more.
2019 medical device security 101 conference.
4th annual medical device cybersecurity risk mitigation conference july 23 24 2019 arlington va.
Ken zalevsky head of medical device cybersecurity bayer.
Managing cybersecurity risk through various assessments robust management systems module 2.
This year s medical device cybersecurity risk mitigation conference will virtually connect security leaders to share best practices in the following modules.